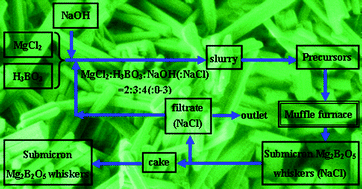
Developing green approaches to micro-/nanomaterials is becoming more and more important in constructing a more sustainable society for chemical as well as materials related community. Here, a byproduct-assisted thermal conversion (BATC) route to submicron Mg2B2O5 whiskers (diameter: 145–350 nm, length: 560–3490 nm) is developed. Room temperature co-precipitation of the reactants leads to precursor slurries, followed by filtration and washing. The resultant precursor containing residual byproduct NaCl is calcined at 800–900 °C for 2 h, giving rise to final submicron Mg2B2O5 whiskers. Compared with the conventional methods for Mg2B2O5 micro-/nanostructures, the present BATC route exhibits distinct advantages, such as improvement of size/morphology uniformity and dispersion of product particles, no need of additionally introducing abundant flux agent, recycling and reuse of byproduct NaCl as flux, no need of boiling water in flux separation and product purification, reduction of waste water rich of NaCl, energy saving and low cost. The present BATC route is high-pressure-free, environmental benign, and thus can be extended for designing novel sustainable approaches to other micro-/nanostructures especially those traditionally acquired by high temperature molten salt synthesis or high pressure supercritical/hydrothermal method.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...