1,4-Bis(1,3-dioxo-2-indenylidene)cyclohexane: polymorphism, gas phase oxidation and enol form mediated radical formation in the solid state†
Abstract
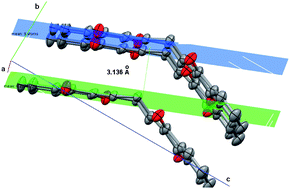
- This article is part of the themed collection: Reactions in molecular solids and host–guest systems

 Please wait while we load your content...
Please wait while we load your content...