Synthesis of anisometric KNbO3 and K0.5Na0.5NbO3 single crystals by chemical conversion of non-perovskite templates
Abstract
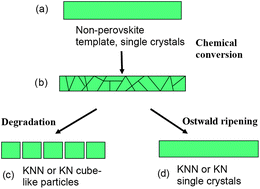
KNbO3 (KN) and K1−xNaxNbO3 (KNN) anisometric crystals have been produced by a chemical conversion route. Crystals of the compounds K4Nb6O17, K2Nb4O11 and KNb3O8 with high aspect ratio were first grown in molten KCl. The non-perovskite compounds were further converted to KN or KNN perovskites by reaction with K2CO3 or Na2CO3 at 800 °C with or without the presence of molten KCl. Through a dissolution–precipitation mechanism needle- or plate-like KNN and KN particles with high aspect ratios were formed with preferred orientation along the [011] or [100] directions. A short reaction time was observed to yield complete chemical conversion to polycrystalline KN/KNN, while single crystals with the morphology resembling the non-perovskite templates were obtained after longer reaction time by

 Please wait while we load your content...
Please wait while we load your content...