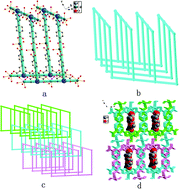
Solvothermal reactions of partially or wholly deprotonated biphenyl-3,4′,5-tricarboxylate (H3L) with nitrates of cadmium, manganese and cobalt, in the presence or absence of auxiliary 4,4′-bipyridine (bipy) and 1-(3,5-di(1H-imidazol-1-yl)phenyl)-1H-imidazole (timp) ligands in H2O–DMF, produce four new complexes, namely, {[Cd3(L)2(H2O)9]·(H2O)5}n (1), {[Cd2(HL)2(timp)(H2O)4]·(H2O)4}n (2), {Mn(HL)(bipy)0.5(H2O)}n (3), {Co(HL)(bipy)0.5(H2O)}n (4). Their structures have been determined by single-crystal X-ray diffraction analysis and further characterized by elemental analysis, IR spectra, and thermogravimetric analysis. Complex 1 possesses a three-dimensional (3D) framework composed of two-dimensional (2D) T-shaped molecular bilayer motifs polycatenated with each other. In complex 2, multicarboxylate ligands and timp ligands link Cd centers to generate one-dimensional (1D) ladder structures which are further connected by π–π interactions to form a 3D supramolecular structure. Complexes 3 and 4 are isostructural and possess 2D networks. Magnetic susceptibility measurements indicate that complexes 3 and 4 exhibit ferromagnetic coupling between adjacent Mn(II) ions and Co(II) ions. The photoluminescent properties of 1 and 2 have been studied in the solid state at room temperature.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...