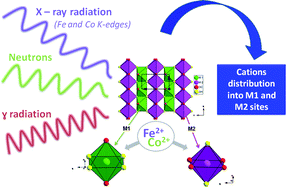
Four samples of the solid solution (Co1−xFex)2(OH)2(C8H4O4) x = 0.25 (1), x = 0.5 (2), x = 0.75 (3); x = 0.88 (4) (C2/m) were investigated by synchrotron radiation using Multi-wavelength Anomalous Diffraction (MAD) and multipattern refinements in order to locate the two neighbour metallic elements Fe and Co. Although there is no cation ordering, the two metallic sites M1 (0, 0, 0) and M2 (0, 0.5, 0.5) are preferentially occupied by Co and Fe respectively. In this article we demonstrate that this distribution is in agreement with neutron data and 57Fe Mössbauer spectra at 100 K. Consequently, the MAD method can be used to locate close d-metallic elements in metal–organic frameworks.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...