Intra- and intermolecular interactions and water pincer in the crystal structure of a 3-P(O)Ph2 substituted 1,2,3,6-tetrahydrophosphinine oxide hydrate†
Abstract
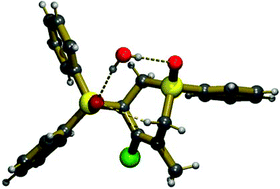
Single crystal X-ray structure analysis of 3-diphenylphosphinoxido-1-phenyl-4-chloro-1,2,3,6-tetrahydrophosphinine oxide (1)·H2O provided experimental proof for a transannular P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯HC type interaction. The embedded
O⋯HC type interaction. The embedded ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the P-heterocycle via intra-associate hydrogen bonds. Theoretical calculations showed that the conformation of the hetero-ring alters only slightly due to the presence of the
O groups of the P-heterocycle via intra-associate hydrogen bonds. Theoretical calculations showed that the conformation of the hetero-ring alters only slightly due to the presence of the

 Please wait while we load your content...
Please wait while we load your content...