Metal-free naphthannulation reactions of yne-allenone esters for accessing polycyclic aromatic hydrocarbons†
Abstract
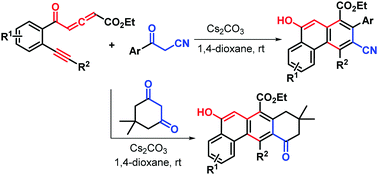
The first metal-free base-promoted naphthannulation reactions of yne-allenone esters were established for the direct assembly of a wide range of polycyclic aromatic hydrocarbons with generally good yields. The naphthannulation reaction of yne-allenone esters with β-ketonitriles provided new phenanthrene-1-carboxylates via a base-mediated [2+2] cycloaddition/ring expansion sequence, whereas hitherto unreported tetracyclic tetrahydrotetraphene-7-carboxylates were obtained with good yield via a sequential double annulation cascade of yne-allenone esters with dimedone as a diphilic reagent.


 Please wait while we load your content...
Please wait while we load your content...