Unexpected high binding energy of CO2 on CH3NH3PbI3 lead-halide organic–inorganic perovskites via bicarbonate formation†
Abstract
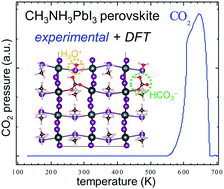
The adsorption kinetics of CO2 was experimentally characterized in ultra-high vacuum (UHV). Unexpectedly, high desorption temperatures (640 K, 170 kJ mol−1) were seen. Density functional theory (DFT) calculations suggest the stabilization mechanism: bicarbonate formation in the defected perovskite film due to CO2 and H2O coadsorption.


 Please wait while we load your content...
Please wait while we load your content...
