TfNHNHBoc as a SCF3 source for the sulfenylation of indoles†
Abstract
An unprecedented use of trifluoromethanesulfonyl hydrazides as effective SCF3 sources has been established in the sulfenylation of indoles. A range of substituted indoles participated in CuCl-catalyzed oxidative sulfenylation reaction with TfNHNHBoc in the presence of dimethyl sulfoxide to furnish structurally diverse 3-indolyl trifluoromethyl thioethers in moderate to good yields with very high regioselectivity.
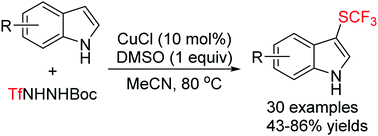


 Please wait while we load your content...
Please wait while we load your content...