Chiral separation of diastereomers of the cyclic nonapeptides vasopressin and desmopressin by uniform field ion mobility mass spectrometry†
Abstract
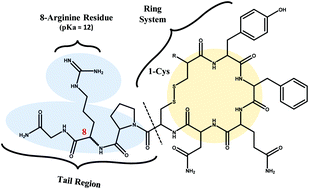
In this study ion mobility-mass spectrometry (IM-MS) is used to distinguish chiral diastereomers of the nonapeptides desmopressin and vasopressin. The differences in gas phase cross sectional area (ca. 2%) were sufficient to directly resolve the enantiomers present in a binary mixture. Results from computational modeling indicate that chiral recognition by IM-MS for nonapeptides is possible due to their diastereomer-specific conformations adopted in the gas-phase, namely a compact ring-tail conformer specific to the L-diastereomer forms.


 Please wait while we load your content...
Please wait while we load your content...