Photooxidation of oxazolidine molecular switches: uncovering an intramolecular ionization facilitated cyclization process†
Abstract
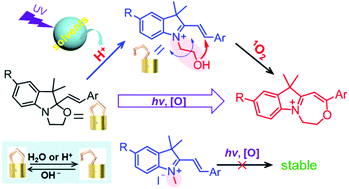
Photooxidation of oxazoline (OXA) molecular switches through media-induced intramolecular ionization is reported. The formation of the photooxidation product occurs by attack of the flexible donor group (–CH2CH2OH) to the adjacent C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C with 1O2 as the oxidant. The novel seven-membered ring sub-structure of the photooxidation product was inferred by HRMS, IR and 1H NMR spectroscopy. Additionally, acid released from solvent photolysis was found to affect the product formation and efficiency of the photooxidation.
C with 1O2 as the oxidant. The novel seven-membered ring sub-structure of the photooxidation product was inferred by HRMS, IR and 1H NMR spectroscopy. Additionally, acid released from solvent photolysis was found to affect the product formation and efficiency of the photooxidation.


 Please wait while we load your content...
Please wait while we load your content...