Dynamic kinetic resolution of bis-aryl succinic anhydrides: enantioselective synthesis of densely functionalised γ-butyrolactones†
Abstract
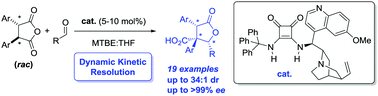
The efficient Dynamic Kinetic Resolution (DKR) of disubstituted anhydrides has been shown to be possible for the first time. Using an ad hoc designed organocatalyst and an enantio- and diastereoselective cycloaddition process with aldehydes, stereochemically complex γ-butyrolactone derivatives can be obtained – with control over three contiguous stereocentres, one of which is all carbon quaternary.


 Please wait while we load your content...
Please wait while we load your content...