Synthesis and antioxidant capacity of novel stable 5-tellurofuranose derivatives†
Abstract
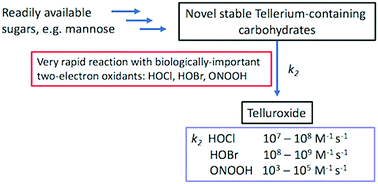
Novel stable tellurium-containing carbohydrate derivatives are prepared from hexitols and pentitols through a double nucleophilic substitution with NaHTe in PEG-400. These tellurosugars react at very high rates with two-electron oxidants, including hypochlorous and peroxynitrous acid, formed at sites of inflammation, and show considerable promise as protective agents against oxidative damage.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...