Glucuronic acid as a helix-inducing linker in short peptides†
Abstract
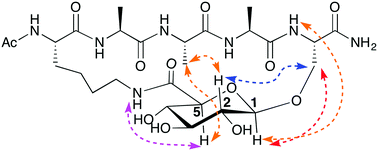
A new strategy is demonstrated for making peptides helical, using a carbohydrate to bridge between sidechains at each end of a pentapeptide. CD and NMR spectra establish that both an α-helix and a 310-helix structure can form depending upon the bridge.


 Please wait while we load your content...
Please wait while we load your content...