A strategy for generating aryl radicals from arylborates through organic photoredox catalysis: photo-Meerwein type arylation of electron-deficient alkenes†
Abstract
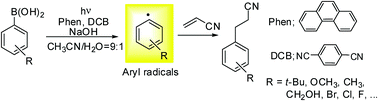
Photoinduced reactions of arylboronic acids with electron deficient alkenes under mild organic photoredox catalysis conditions lead to the formation of Meerwein arylation type adducts via the generation of aryl radicals.
- This article is part of the themed collection: Editor’s Choice: Main group reagents and catalysts in organic reactions


 Please wait while we load your content...
Please wait while we load your content...