Fluorescence spectroscopy reveals N-terminal order in fibrillar forms of α-synuclein†
Abstract
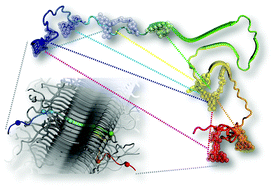
The neuronal protein α-synuclein (αS) plays a key role in Parkinson's disease, forming inclusions termed Lewy bodies and Lewy neurites. Recent improvements in cryo-electron diffraction and solid state NMR (ssNMR) have led to the elucidation of the structures of peptides derived from the αS fibril core and full-length human αS in fibrils. Despite the valuable insight offered by these methods, there are still several questions about the structures’ relevance to pathological aggregates. Herein, we present fluorescence data collected in vitro under the conditions which fibrils are typically assembled. Our data suggest that, in solution, fibrils are largely structured as observed by ssNMR. However, we observe significant disparities in the αS N-terminus as compared to ssNMR data, which provide insight on its important role in αS aggregation and fibril structure.


 Please wait while we load your content...
Please wait while we load your content...