Transition metal free regio-selective C–H hydroxylation of chromanones towards the synthesis of hydroxyl-chromanones using PhI(OAc)2 as the oxidant†
Abstract
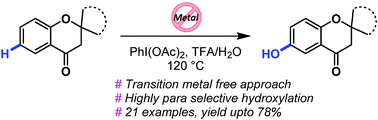
The chromanone scaffold is considered as a privileged structure in drug discovery. Herein, we report a highly efficient PhI(OAc)2 mediated regioselective, direct C–H hydroxylation of chromanones. This method offers easy access to substituted 6-hydroxy chromanones in moderate to good isolated yields, thus paving the way for their pharmaceutical studies.


 Please wait while we load your content...
Please wait while we load your content...