Total synthesis of (+)-brasilenyne via concise construction of an oxonane framework containing a 1,3-cis,cis-diene†
Abstract
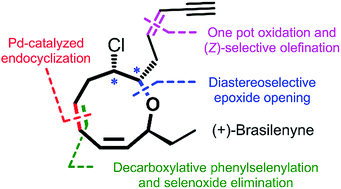
The enantioselective total synthesis of (+)-brasilenyne has been accomplished. The key features of the synthesis include the convergent preparation of a highly functionalized endocyclization precursor via selective epoxide opening, the construction of an oxonene skeleton through perfect regioselective Pd(0)-catalyzed endocyclization, and the installation of a 1,3-cis,cis-diene unit via a decarboxylative photophenylselenylation and site-selective selenoxide elimination sequence.
- This article is part of the themed collection: Natural product synthesis


 Please wait while we load your content...
Please wait while we load your content...