Rhodium-mediated asymmetric transfer hydrogenation: a diastereo- and enantioselective synthesis of syn-α-amido β-hydroxy esters†
Abstract
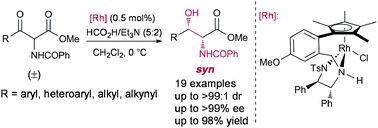
The preparation of syn α-benzoylamido β-hydroxy esters through asymmetric transfer hydrogenation (ATH) with a tethered Rh(III)–DPEN complex via dynamic kinetic resolution (DKR) has been developed for the first time starting from α-benzoylamido β-keto esters. A variety of α-benzoylamido β-keto esters were converted under mild conditions into the corresponding syn α-benzoylamino β-hydroxy esters with high yields (up to 98%) and diastereomeric ratios (up to >99 : 1 dr) as well as excellent enantioselectivities (up to >99% ee).


 Please wait while we load your content...
Please wait while we load your content...