N-Gemini peptides: cytosolic protease resistance via N-terminal dimerization of unstructured peptides†
Abstract
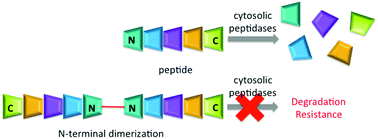
Herein we describe a synthetically simple strategy for increasing the lifetime of unstructured peptides in cytosolic environment via dimerization at the N-terminus to block threading into the catalytic cleft of cytosolic proteases. We establish this approach with kinase substrates, allowing for phosphorylation in cells as a demonstration of protease resistance.


 Please wait while we load your content...
Please wait while we load your content...