Tunable plasmon resonance of molybdenum oxide nanoparticles synthesized in non-aqueous media†
Abstract
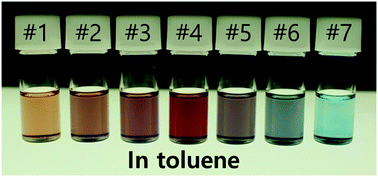
Plasmonic compound nanoparticles (NPs) have attracted great interest because they are prepared at lower cost and show unique optical properties. However, full replacement of the plasmonic noble metal NPs with the compound NPs has been difficult because most of the compound NPs exhibit plasmon resonance in the infrared range owing to low free carrier density and mobility. In order to overcome this limitation, we developed a new synthetic method for plasmonic MoO2 and MoO3−x NPs. Those NPs exhibit plasmon resonance at ∼500 nm and 600–1000 nm, respectively, likely because of high carrier densities. The plasmonic properties of the NPs are tunable by changing the synthetic conditions or oxidizing and reducing the NPs. Their refractive index sensitivities are 115–260 nm RIU−1. Those molybdenum oxide NPs are expected to substitute for plasmonic noble metal NPs in optical, electronic, sensing and light harvesting devices and materials.


 Please wait while we load your content...
Please wait while we load your content...