Efficient Ni-catalyzed conversion of phenols protected with 2,4,6-trichloro-1,3,5-triazine (TCT) to olefins†
Abstract
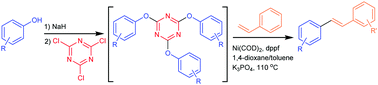
An efficient Ni(COD)2/dppf catalyzed olefination of Ar–O–TCT as synthetic equivalents of aryl halides is described. Activation of C–O bonds in phenols as readily available compounds was achieved with 2,4,6-trichloro-1,3,5-triazine (TCT). This method provides practical access to 1,2-disubstituted olefins in high yields and high functional group compatibility.


 Please wait while we load your content...
Please wait while we load your content...