Probing the reaction interface in Li–O2 batteries using electrochemical impedance spectroscopy: dual roles of Li2O2†‡
Abstract
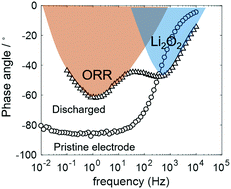
Li2O2 is revealed to play a positive role by facilitating the kinetics of the oxygen reduction reaction in aprotic Li–O2 batteries, in addition to the well-known negative role by passivating the electrode surface. Moreover, it is inferred that the oxygen reduction reaction takes place at the Li2O2/electrolyte interface, rather than the electrode/Li2O2 interface, in our cell.


 Please wait while we load your content...
Please wait while we load your content...