Recent developments in transition-metal photoredox-catalysed reactions of carbonyl derivatives
Abstract
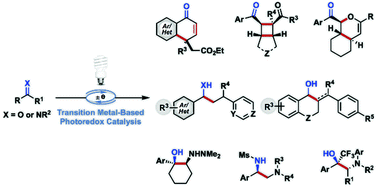
Single-electron reduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds of aldehydes, ketones, and imines results in the formation of ketyl and α-aminoalkyl anion radicals, respectively. These reactive intermediates are characterized by an altered electronic character with respect to their parent molecules and undergo a diverse range of synthetically useful transformations, which are not available to even-electron species. This Review summarizes the reactions of ketyl and α-aminyl radicals generated from carbonyl derivatives under transition-metal photoredox-catalysed conditions. We primarily focus on recent developments in the field, as well as give a brief overview of catalytic enantioselective transformations that provide a means to achieve precise stereocontrol over the reactivity of ion radicals.
N bonds of aldehydes, ketones, and imines results in the formation of ketyl and α-aminoalkyl anion radicals, respectively. These reactive intermediates are characterized by an altered electronic character with respect to their parent molecules and undergo a diverse range of synthetically useful transformations, which are not available to even-electron species. This Review summarizes the reactions of ketyl and α-aminyl radicals generated from carbonyl derivatives under transition-metal photoredox-catalysed conditions. We primarily focus on recent developments in the field, as well as give a brief overview of catalytic enantioselective transformations that provide a means to achieve precise stereocontrol over the reactivity of ion radicals.


 Please wait while we load your content...
Please wait while we load your content...