Effect of N- and C-terminal functional groups on the stability of collagen triple helices†
Abstract
The effect of charged versus neutral N- and C-termini on the stability of the collagen triple helix was examined. Thermal denaturation studies at different pH with collagen model peptides showed that an ammonium group at the N-terminus destabilizes the triple helix more than a carboxylate at the C-terminus. A neutral carboxylic acid stabilizes the triple helix more than an amido moiety at the C-terminus.
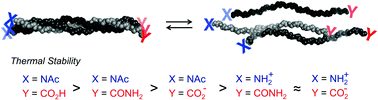


 Please wait while we load your content...
Please wait while we load your content...