Dynamic protein self-assembly driven by host–guest chemistry and the folding–unfolding feature of a mutually exclusive protein†
Abstract
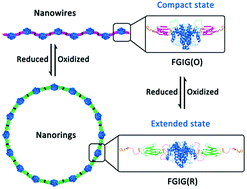
A novel exploration utilizing a well-designed fusion protein containing a redox stimuli-responsive domain was developed to construct dynamic protein self-assemblies induced by cucurbit[8]uril-based supramolecular interactions. The reversible interconversion of the morphology of the assemblies between nanowires and nanorings was regulated precisely by redox conditions.


 Please wait while we load your content...
Please wait while we load your content...