Closed-shell paramagnetic porphyrinoids†
Abstract
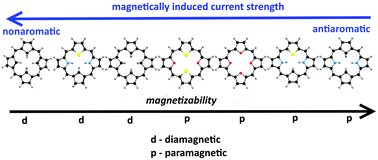
Magnetizabilities and magnetically induced ring-current strength susceptibilities have been calculated at the Hartree–Fock, density functional theory and second order Møller–Plesset levels for a number of antiaromatic closed-shell carbaporphyrins, carbathiaporphyrins and isophlorins. The calculations yield a linear relation between magnetizabilities and ring-current strength susceptibilities. The calculations show that the porphyrinoids with the largest ring-current strength susceptibility are closed-shell paramagnetic molecules with positive magnetizabilities. The closed-shell paramagnetism is due to the large paramagnetic contribution to the magnetizability originating from the strong paratropic ring current in the antiaromatic porphyrinoids.


 Please wait while we load your content...
Please wait while we load your content...