A kinetic study of mechanochemical halogen bond formation by in situ31P solid-state NMR spectroscopy†
Abstract
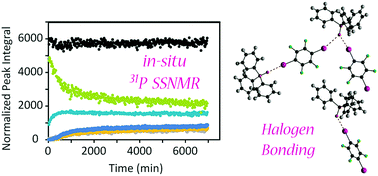
In situ 31P solid-state NMR studies of halogen bond formation between triphenylphosphine oxide and para-diiodotetrafluorobenzene provide insights into the cocrystallisation process and provide an estimate of the activation energy. The effects of temperature, magic-angle spinning speed, and initially added liquid on the reaction rate and mechanism have been investigated.
- This article is part of the themed collections: 1st International Conference on Noncovalent Interactions and Commemorating Michael Faraday (1791-1867)


 Please wait while we load your content...
Please wait while we load your content...