Sulfur-directed carbon–sulfur bond cleavage for Rh-catalyzed regioselective alkynylthiolation of alkynes†
Abstract
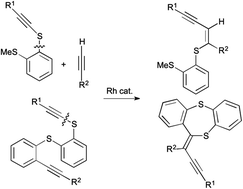
Sulfur-directed sp C–S bond cleavage along with a regioselective reaction with alkynes proceeded to give (Z)-enyne sulfides in high to excellent yields. Mechanistic studies were conducted, including characterization of an intermediate. An intramolecular variant realized the construction of a dibenzodithiepin skeleton, which is a seven-membered ring with two sulfur atoms.


 Please wait while we load your content...
Please wait while we load your content...