Electrocatalytic water oxidation by Cu(ii) ions in a neutral borate buffer solution†
Abstract
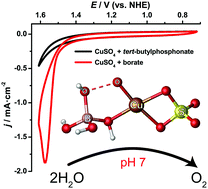
Herein we report that a Cu(II) salt can efficiently catalyze water oxidation in a neutral borate buffer. Studies on the mechanism revealed that the borate anion serves as an oxygen donor in O–O bond formation during the process of water oxidation, assisting in enhancing the catalytic activity of Cu(II).


 Please wait while we load your content...
Please wait while we load your content...