Determining rotational dynamics of the guanidino group of arginine side chains in proteins by carbon-detected NMR†
Abstract
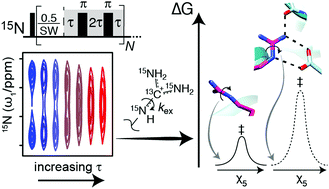
Arginine residues are imperative for many active sites and protein-interaction interfaces. A new NMR-based method is presented to determine the rotational dynamics around the Nε–Cζ bond of arginine side chains. An application to a 19 kDa protein shows that the strengths of interactions involving arginine side chains can be characterised.


 Please wait while we load your content...
Please wait while we load your content...