Copper-catalyzed trifluoromethylazidation and rearrangement of aniline-linked 1,7-enynes: access to CF3-substituted azaspirocyclic dihydroquinolin-2-ones and furoindolines†
Abstract
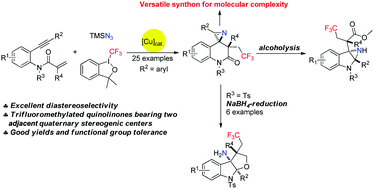
A set of reactions involving copper-catalyzed trifluoromethylazidation and then rearrangement of aniline-linked 1,7-enynes with the relatively poorly reactive Togni reagent I and TMSN3 was developed, and provided facile access to structurally diverse and useful CF3-substituted azaspirocyclic dihydroquinolin-2-ones bearing two adjacent quaternary carbon centers. We were also able to obtain these products on a large scale. Moreover, the obtained products were further transformed into a range of synthetically valuable furoindolines bearing three consecutive quaternary carbon centers after reduction by NaBH4. Also, the delivery of multi-functionalized aziridines as a result of alcoholysis and LiAlH4-reduction of corresponding dihydroquinolin-2-ones 2ba demonstrated the synthetic value of this newly developed protocol.


 Please wait while we load your content...
Please wait while we load your content...