Gold-catalyzed diastereoselective domino dearomatization/ipso-cyclization/aza-Michael sequence: a facile access to diverse fused azaspiro tetracyclic scaffolds†
Abstract
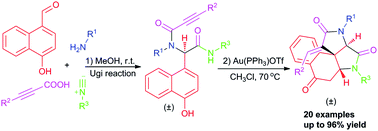
A facile and diversity-oriented access to complex tetracyclic benzo[e]pyrrolo[2,3-c]indole-2,4,7(5H)-triones through a post-Ugi gold(I)-catalyzed domino dearomatization/ipso-cyclization/aza-Michael sequence is elaborated. This process furnishes tetracyclic scaffolds in good yields from readily available precursors with unique diastereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...