Rh(iii)-Catalyzed bilateral cyclization of aldehydes with nitrosos toward unsymmetrical acridines proceeding with C–H functionalization enabled by a transient directing group†
Abstract
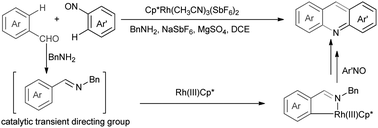
A Rh(III)-catalyzed bilateral cyclization was developed for the efficient construction of acridines proceeding with C–H functionalization whereby in situ formation and removal of an imino transient directing group in the presence of catalytic amount of BnNH2 are achieved. In this transformation, a sequential Rh(III)-catalyzed C–H amination, cyclization, and aromatization process was involved.


 Please wait while we load your content...
Please wait while we load your content...