Controlling selectivity in the Ullmann reaction on Cu(111)†
Abstract
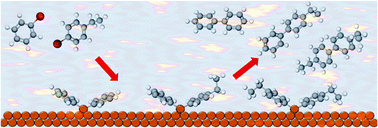
Using a surface science approach, the selectivity in the Ullmann cross-coupling of aryl halides on Cu(111) has been understood and controlled. The binding strength of the reactants and repulsion between them dictates which organometallic intermediates form, and hence the product distribution. Cross coupling can be maximized at low reactant concentrations.


 Please wait while we load your content...
Please wait while we load your content...