Computational design of cephradine synthase in a new scaffold identified from structural databases†
Abstract
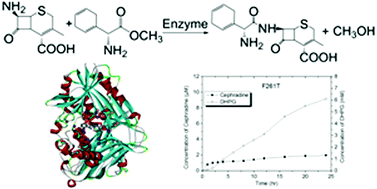
Computational enzyme design exhibits excellent performance for identifying potential scaffolds from structural databases and creating new enzymatic catalysts from naught. Using the active site-matching algorithm ProdaMatch, we identified a new scaffold cocaine esterase from Rhodococcus sp. that showed modest activity (kcat/Km = 0.018 M−1 s−1) towards the hydrolysis of β-lactam antibiotic cephradine. The identified cocaine esterase scaffold afforded low sequence identity (<30%) with the known β-lactam synthases, such as penicillin G acylase or α-amino acid ester hydrolase, and was able to catalyze the condensation reaction between D-dihydrophenylglycine methyl ester and 7-aminodesacetoxycephalosporanic acid to produce cephradine via a kinetically controlled synthesis. By virtue of the computational enzyme design protocol, hundreds of sequences were predicted in the cocaine esterase scaffold to promote the catalytic activity towards the hydrolytic reaction of cephradine. Moreover, a single mutant (F261T) was experimentally confirmed to have improved the catalytic efficiency by ten times (kcat/Km = 0.193 M−1 s−1), indicating that the novel scaffold cocaine esterase may be potentially redesigned to become an industrially useful cephradine synthase.


 Please wait while we load your content...
Please wait while we load your content...