Nucleoside-linked shunt products in the reaction catalyzed by the class C radical S-adenosylmethionine methyltransferase NosN†
Abstract
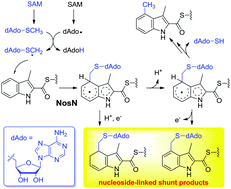
NosN is a class C radical S-adenosylmethionine (SAM) methyltransferase (RSMT) involved in the biosynthesis of nosiheptide, a clinically interesting thiopeptide antibiotic produced by Streptomyces actuosus. NosN employs an unprecedented catalytic mechanism, in which SAM is converted to 5′-methylthioadenosine (MTA) as a direct methyl donor. In this study, we report identification of several nucleoside-linked shunt products in the NosN-catalyzed reaction. Comparative analysis of NosN and the class A RSMT RlmN and further density functional theory (DFT) calculations reveal important mechanistic insights into the catalyses of the two types of enzymes, showing that the radical intermediates generated by similar pathways can have very diverse reactivities. This investigation provides strong evidence supporting the previous mechanistic proposal of NosN catalysis, validating the presence of a key radical adduct that results from the addition of an MTA-derived methylene radical onto the C4 of the indolyl substrate.


 Please wait while we load your content...
Please wait while we load your content...