A transition-metal-free fast track to flavones and 3-arylcoumarins†
Abstract
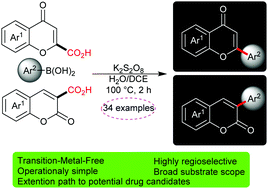
A highly regioselective and transition-metal free one-pot arylation of chromenones with arylboronic acids has been achieved employing K2S2O8. The procedure consists of a sequence of some reactions including an arylation/decarboxylation cascade and proceeds well in aqueous media to afford biologically interesting flavones and 3-arylcoumarins. This method exhibited excellent selectivity and functional group tolerance under mild conditions. The reaction also showed perfect efficacy for the preparation of styryl coumarins.


 Please wait while we load your content...
Please wait while we load your content...