Facile synthesis of chlorin bioconjugates by a series of click reactions†
Abstract
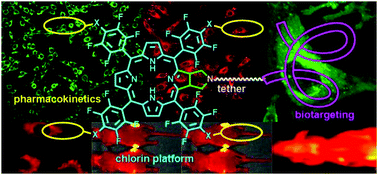
A multifunctional chlorin platform appended with four short polyethylene glycols and a carboxylate-linker allows rapid conjugation to biotargeting motifs such as proteins and oligonucleotides. The stability and photophysical properties of the chlorin enable development of diagnostics, imaging, molecular tracking, and theranostics.


 Please wait while we load your content...
Please wait while we load your content...