Gold-catalyzed intermolecular cyclocarboamination of ynamides with 1,3,5-triazinanes: en route to tetrahydropyrimidines†
Abstract
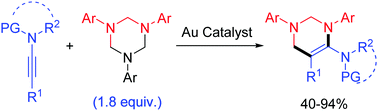
Gold-catalyzed regioselective cyclocarboamination of ynamides with 1,3,5-triazinanes opens a facile and modular access to valuable 5-aminotetrahydropyrimidines in good to excellent yields. It constitutes an unprecedented yet challenging annulation of ynamides with unstrained saturated heterocycles. This new protocol is distinguished by easy operation, readily available starting materials, stable four-atom building units, good functional-group compatibility and scaling-up potential. The preliminary mechanistic studies indicate that the present intermolecular cyclocarboamination arises from a pseudo-three-component [2+2+2] cycloaddition.


 Please wait while we load your content...
Please wait while we load your content...