Solvent-free, mechanochemical syntheses of bulk trihalide perovskites and their nanoparticles†
Abstract
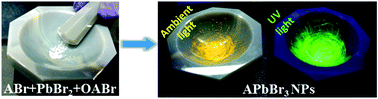
For the first time, we have synthesized APbBr3 (A = Cs+/MA+/FA+, where MA+ = CH3NH3+ and FA+ = CH(NH2)2+) bulk as well as nanoparticles (NPs) by solid-state reactions at room temperature. This facile strategy yields different shape structures e.g. square and rectangular (CsPbBr3), spherical (MAPbBr3) and parallelogram (FAPbBr3) NPs.


 Please wait while we load your content...
Please wait while we load your content...