Metal-free nitroxyl radical-mediated β-C(sp3)–H amination of saturated ketones with heteroaryl halides: multiple roles of TEMPO†
Abstract
The first metal-free nitroxyl-radical-mediated β-amination of saturated ketones by using heteroaryl halides as amide precursors has been developed. This reaction proceeds through a cascade α-aminoxylation/Cope-like elimination/aza-Michael addition sequence to afford β-amino ketone derivatives with excellent yields. TEMPO plays multiple roles in the current β-amination process, including those of an oxidant, an α-aminoxylation reagent, a β-hydrogen acceptor, an in situ base, and an oxygen source.
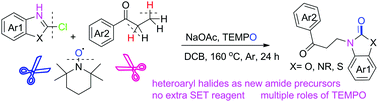


 Please wait while we load your content...
Please wait while we load your content...