C(sp3)–H dehydrogenation and C(sp2)–H alkoxy carbonylation of inactivated cyclic amines towards functionalized N-heterocycles†
Abstract
A novel and efficient synthesis of tetrahydropyridine (THP)-, dihydropyrrole (DHP)-, or tetrahydroazepine (THA)-3-carboxylates via cascade reactions of inactivated cyclic amines with CO and alcohols is presented. To our knowledge, this should be the first example in which functionalized N-heterocycles were prepared directly from Pd-catalyzed C(sp3)–H dehydrogenation and C(sp2)–H carbonylation of saturated cyclic amines. Moreover, the DHP-3-carboxylates thus obtained could readily undergo an oxidative aromatization to give pyrrole-3-carboxylates by using O2 as a green oxidant. Notable features of the methods developed herein include simple substrates, high efficiency and excellent atom-economy, mild reaction conditions, and broad substrate scope.
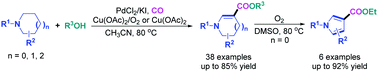


 Please wait while we load your content...
Please wait while we load your content...