Symmetry breaking above room temperature in an Fe(ii) spin crossover complex with an N4O2 donor set†
Abstract
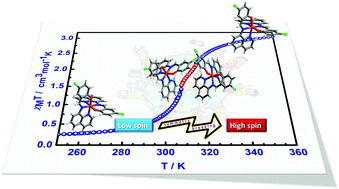
[Fe(qsal-Cl)2] is one of the few known Fe(II) spin crossover compounds with an N4O2 donor atom environment. It shows an abrupt two-step spin transition at 308 and 316 K and importantly, symmetry breaking at the highest temperature reported, to date, in spin crossover compounds.


 Please wait while we load your content...
Please wait while we load your content...