Direct enantioselective C-acylation for the construction of a quaternary stereocenter catalyzed by a chiral bicyclic imidazole†
Abstract
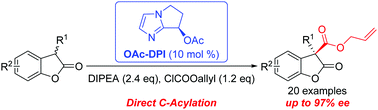
A direct enantioselective C-acylation of 3-substituted benzofuran-2(3H)-ones (with up to 97% ee) was developed using a chiral bicyclic imidazole catalyst OAc-DPI and an achiral tertiary amine additive DIPEA. The reaction mechanism for the direct C-acylation has been investigated using both experimental and theoretical studies.


 Please wait while we load your content...
Please wait while we load your content...