B(C6F5)3-Catalyzed transfer 1,4-hydrostannylation of α,β-unsaturated carbonyls using iPr-tricarbastannatrane†
Abstract
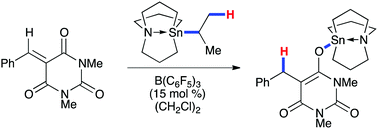
Tris(pentafluorophenyl)borane, B(C6F5)3, has been found to be an effective catalyst to access the hydridoborate anion, [N(CH2CH2CH2)3Sn][HB(C6F5)3], via hydride abstraction from the hypercoordinated tin reagent, iPr-tricarbastannatrane. This process has been applied to the B(C6F5)3-catalyzed transfer 1,4-hydrostannylation of electron-deficient olefins, namely benzylidene barbituric acids. Insights into the mechanism have been obtained via a series of 1H, 2H, 11B, 13C, and 119Sn NMR spectroscopy, mass spectrometry, and labeling experiments.


 Please wait while we load your content...
Please wait while we load your content...