Redox interactions of Au(iii) with carboxylated dithiafulvenes and tetrathiafulvalene analogues in polar organic media†
Abstract
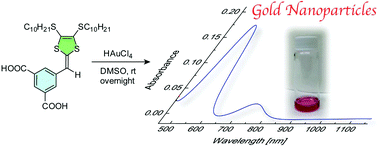
The redox interactions between HAuCl4 and a series of π-conjugated organic donors, namely carboxylated dithiafulvenes and tetrathiafulvalene vinylogues, were investigated. Interestingly, the dithiafulvene derivative with two carboxylic groups showed the ability to directly induce the formation of Au(0) nanoparticles in dipolar aprotic solvents such as DMF and DMSO.

 Please wait while we load your content...
Please wait while we load your content...