Radical trideuteromethylation with deuterated dimethyl sulfoxide in the synthesis of heterocycles and labelled building blocks†
Abstract
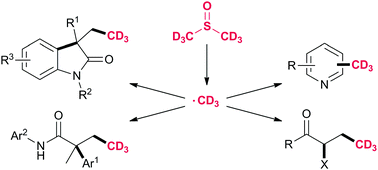
The potential of deuterated pharmaceuticals is being widely demonstrated. Here we describe the first trideuteromethylation under radical reaction conditions using deuterated dimethyl sulfoxide as a reagent for the synthesis of labelled heterocycles and trideuteromethylated compounds. A broad scope of the developed method for the synthesis of various scaffolds was demonstrated.

 Please wait while we load your content...
Please wait while we load your content...