A general and versatile fluorescence turn-on assay for detecting the activity of protein tyrosine kinases based on phosphorylation-inhibited tyrosyl oxidation†
Abstract
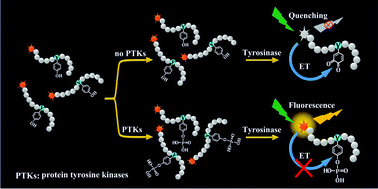
A simple, homogeneous and generic method for detecting protein tyrosine (Tyr) kinase activity is developed based on a tyrosinase-assisted fluorescence turn-on strategy. The tyrosinase-mediated oxidation of the Tyr residue in a fluorescently-labeled peptide may lead to efficient fluorescence quenching, while the tyrosine kinase-catalyzed phosphorylation of the peptide can prevent the Tyr oxidation and thus maintain strong fluorescence.

 Please wait while we load your content...
Please wait while we load your content...