Palladium catalyzed/silver tuned selective mono-/tetra-acetoxylation of o-carboranes via B–H activation†
Abstract
A palladium catalyzed/silver tuned selective mono- and tetra-acetoxylation of o-carboranes has been developed, and a series of mono- and tetra-acetoxylated o-carboranes decorated with active groups have been synthesized with moderate to good yields as well as excellent selectivity. A mechanism involving electrophilic palladation and cyclopalladation of the B–H bond was proposed.
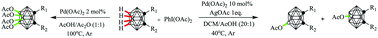

 Please wait while we load your content...
Please wait while we load your content...